EMERGING TECHNOLOGIES: Rexam Coextruded Film Takes Run at Coated, Non-woven Medical Device Packaging Seals
By Steve Mogensen, Allied Development Corp.
When it comes to sealing technology for medical device packaging, coated non-woven top-web constructions remain the standard for performance despite past attempts by coextruded film manufacturers to create the required seal at a lower cost. A recently commercialized coextruded film technology, however, that distinguishes the seal function from the peel mechanism is aiming to become the new peel system of choice.
Table of Contents
Seal Characteristics
Sealant Formulation
New Approach Using Coextruded Film
Sealing Independent of Peeling
Medical device packages typically include a sealant-coated, non-woven, flexible material (i.e., paper or Dupont Tyvek) for one web, and a polymer film as the opposing web. The selection of these materials is important, but the most critical characteristics of the package are created when the seal between the two materials is produced.
Sealing technology is paramount to the packaging industry in general, but nowhere does this technology draw as much attention as it does in medical device packaging. Medical packaging is one of the few packaging segments where easy-opening packages are used so extensively and so effectively. Packaging engineers have long been challenged to create a sealed medical device package that meets all of the requirements of this demanding application.
Seal Characteristics
The seal characteristics that are desired for a medical device package are numerous. Here is a breakdown of the necessary characteristics:
Easy open or easy peel. The force required to open the package is the single most important characteristic. Easy-open or easy-peel seals are a requirement for many device packages, and it is one that the industry has taken seriously. Packages sold as "easy-open" in other markets, such as cereal, are often very inconsistent. This is not the case in device packaging, where easy-open seals are supplied consistently.
Hermetic seal. A consistent easy-open seal is an even greater accomplishment considering the fact that the package must be hermetically sealed and must remain hermetically sealed. If the seal integrity is compromised, sterility is lost — and so is the product. To put this in perspective, a lock seal might take 10 pounds of force (per one lineal inch of seal) to open. An easy-open seal, for example, takes only one pound of force. The margin for error to maintain a hermetic seal and also provide an easy-peel seal is quite small.
Guard against contamination. Device packages are often opened in a sterile environment where it is pertinent that the act of opening the package does not cause contamination. The possibility of generating particulate matter or fiber is particularly worrisome when paper is used as part of the package.
Paper fibers might easily be released into the air when, for example, a package consisting of paper sealed to a film is opened. The formulation of the paper can have an impact on the amount of fibers released, but it is generally accepted that the sealant coating comes apart during the opening process and minimizes the chance of releasing foreign material into the environment.
Proper adhesion to substrate. A similar but additional point is that the adhesive must adhere properly to the substrate so that any tendency for the coating to "flake-off" or "powder" during converting and packaging operations does not occur. It is extremely important that no coating particles contaminate the product being packaged. The proper level of adhesion of the coating to the substrate also makes it possible for the final package to have the desired bond strengths as well as acceptable bond strengths after sterilization.
Tamper evidence. Device packaging must show evidence of tampering, a characteristic that the coatings also provide. If a package is opened and sterility is compromised, this must be readily apparent to the user. Sealants provide this information in two ways. First, a seal that has been opened looks different; it changes from clear to cloudy and remains cloudy. Second, any attempts to reseal the package to its original condition and appearance will fail.
Adequate porosity. Paper and non-wovens are used to create porosity so medical device packages can be ethylene oxide (EtO) sterilized. However, the paper and non-wovens are usually coated with sealant, which could ruin the package's porosity characteristics. The non-thermoformed layer is where the coating is applied, so the thermoforming process does not damage the coating.
However, the paper or non-woven material must be coated in such a way that adequate porosity is maintained for EtO sterilization. This can be accomplished to a certain extent by formulating the coating to allow for gas permeation. The most effective method is to grid coat, applying the coating in a dot-matrix pattern. This leaves a substantial amount of uncoated area for porosity, but at the same time provides the needed sealing characteristics.
Sealant Formulation
The sealant also controls the parameters under which the seal can be made. As previously mentioned, the importance of peel force in the sealed package is critical. The parameters of the process used to create the seal are also very important, and the formulation of the sealant is used to control these parameters as well.
In other words, the sealant formulation determines the temperature, pressure and dwell-time settings used on the equipment used to create the seal.
The formulation goal, therefore, is to create a sealant that provides the desired peel force characteristics in the finished package, but does so with the widest range of settings of temperature, pressure and dwell-time. This is because the wider the range of settings that can be used to create the desired peel force, the easier it is for the seal maker to create a seal with the desired peel force and do it consistently. It is also desirable to create a sealant that requires the lowest possible temperature and pressure settings to obtain the desired peel force.
New Approach Using Coextruded Film
There is a long history of introducing coextruded films into the market that were intended to create the required seal performance at a lower packaging cost. The lower cost is accomplished by eliminating the need to coat the top web. Despite many attempts and limited success in some applications, coated top-web still remains the standard for performance.
Rexam Medical Packaging (Mundelein, IL) has recently commercialized what it claims is a new approach to utilizing a coextruded film to address this market. The technology is called Core-Peel, and according to Dan Penny, film product manager at Rexam, "the basic concept is somewhat unusual because the sealing layer and the peeling layer are distinct layers. Normally the peel and seal layers are the same layer."
Here is how Core-Peel works:
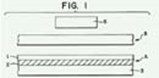
The process starts with a sealing machine, represented by item 6 in Figure 1, a non-woven film (item B), and a Core-Peel film (item A).
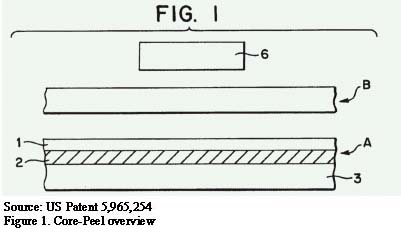
The seal is made and results in the configuration shown in Figure 2, where the seal layer of the Core-Peel film (A) has penetrated into the non-woven film (B).
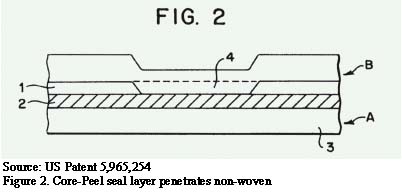
When the package is opened and the seal is separated, the results are shown in Figure 3. The seal layer (1) tears through, and the peel layer (2) peels by cohesive failure.
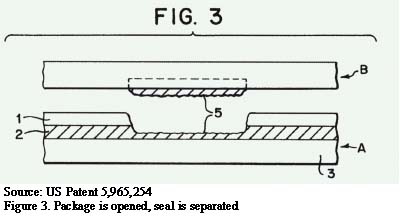
The key layers (shown in Figure 3) are the seal layer (1) and the peel layer (2). Typically the seal layer is constructed mostly of polyethylene, although an incompatible material might be added to make the seal layer more brittle. Some brittleness can assist with breakthrough to the peel layer. The peel layer is usually constructed mostly of polyethylene with a small percentage of filler added, such as talc, to create the cohesive failure.
Sealing Independent of Peeling
According to Penny, "This system is designed to allow the sealing characteristics to be designed independently of the peeling characteristics. In other words, the seal layer can be designed to provide good sealing characteristics, and the peel layer can be designed to provide good peeling characteristics."
The potential advantages of Core-Peel technology are:
• The desired peel strength can be provided consistently over a wide range of sealing parameters because the peel layer fails cohesively. Cohesive failure is at least partially independent of sealing temperature.
• Porosity is maintained because the top web is not coated.
• Clean peel and tamper evidence are provided.
• The temperature, pressure and dwell-time to create a hermetic seal can be minimized.
Only time will tell if this system will displace coated, non-woven top-web as the peel system of choice. Rexam has commercialized the technology for uncoated Tyvek pouches and rollstock for pouches. The company plans to expand the concept to formable films by this summer and to uncoated paper by the end of 2000.
For more information: Rexam Medical Packaging, Tel: 847-362-9000, Fax: 847-918-4665.
About the Author: Steve Mogensen is vice president of sales and marketing for Allied Development Corp., a Lakeville, MN, company that provides a variety of services to the packaging industry aimed at helping companies improve their revenue. Steve joined the startup company in 1996 after more than 20 years experience as a business and technology executive for Viskase Corp., Rexam PLC and the Graphic Packaging Division of ACX Technologies Inc. A Registered Professional Engineer, Mr. Mogensen has a B.S. in Engineering from the University of Illinois and an MBA from the University of Chicago/Lewis University. He can be reached at Tel: 952-898-1832 or Email: sam@allied-dev.com.
