Clinical Project Management
Marken’s intensive project management approach means that your project moves seamlessly through the clinical trial process.
Marken’s intensive project management approach means that your project moves seamlessly through the clinical trial process.
We provide services beginning with the receipt of your clinical trial supplies, continuing on to Pick, Pack & Distribution, and then to collection, reconciliation, storage, and destruction of returns. At Marken, we practice a structured approach to project management:
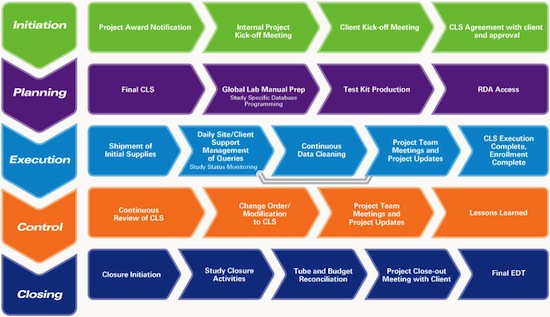
Every Marken customer has a single point of contact during the lifespan of their project: the Global Project Director. The Global Project Director provides global oversight of trials run and is tasked with the overall satisfaction of Marken’s performance across all trials. Additionally, each project has a Regional Project Leader, who manages day-to-day study activities and progress reporting.
To find out more about how Marken’s project management solutions can help your clinical trial process, email info@marken.com or fill out our short contact form:
